Article found from the EWG which can be found clicking HERE
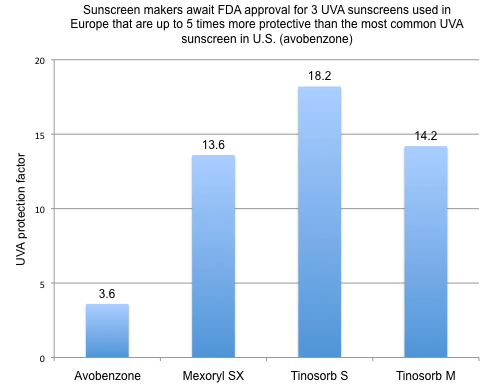
Sunscreen makers can use any of 27 sunscreen chemicals in Europe but only 17 in the United States (Osterwalder 2010). Seven approved compounds that absorb UVA radiation are available in Europe, only three in the U.S. Among those approved in Europe are three – Tinosorb S, Tinosorb M and Mexoryl SX – that are between 3.8 times and 5.1 times more protective than avobenzone, the most common UVA filter in the U.S. (see figure).
Five years ago companies began to apply for FDA approval to use some of these compounds. They are still waiting.
An EWG analysis of this year’s products – specifically, 530 beach and sport sunscreens with SPF ratings of 30+ – found that more than 60 percent of them provide inadequate UVA protection. Those 330 products are too weak for the European market, where manufacturers voluntarily comply with a European Union recommendation that all sunscreens provide meaningful UVA protection in relation to the sunburn protection factor (SPF), a measure of the product’s ability to shield against UVB rays (European Commission 2006).

Source: EWG analysis of UV protection factor using standard industry sunscreen model (BASF 2010), assuming percentage of active ingredient in product equal to maximum allowable amount, or the concentration a company requested that FDA approve for use in sunscreen in the company’s submitted Time and Extent Application.
CIBA Specialty Chemicals Inc. applied in April 2005 for approval of Tinosorb S and Tinosorb M. Sunscreens in Europe have contained these compounds for a decade; FDA has not yet acted.
Loreal submitted an application for Mexoryl SX in September 2007. FDA has not approved the compound for general use in sunscreens but did approve it for use in a small number of specific sunscreens sold under Loreal’s “LaRoche-Posay” brand. Mexoryl SX has been on the market in Europe since 1991.
The upshot of FDA’s delays is that Americans have fewer choices and notably poorer UVA protection than is available in Europe.
Tinosorb S and Tinosorb M offer stable, broad-spectrum protection and appear to be much better UVA blockers than avobenzone. They penetrate the skin in insignificant amounts, pose fewer potential health risks and possess no known hormone-disrupting properties, unlike ingredients in common U.S. sunscreens. Unless FDA approves them, not a single sunscreen sold in the U.S. will earn FDA’s four-star top rating for UVA protection under the system proposed in the agency’s draft sunscreen regulations, based on a standard industry model for rating sunscreen efficacy (BASF 2010).
In Europe as in the US, UVA regulations have not been finalized. But Europe’s proposed standards for UVA protection are far more stringent than FDA’s. The agency has spent years finalizing a rule that would merely require disclosure of UVA protection levels, while Europe has proposed that sunscreens provide UVA protection at a level at least one-third as strong as the sunburn protection level (SPF) (European Commission 2006).
This means the minimum UVA protection in Europe would be roughly equivalent to FDA’s proposed three-star protection level. Requiring balanced protection across the UVB and UVA spectrum has the secondary effect of limiting sky-high SPF values, ensuring that sunburn protection isn’t out of step with protection from other health problems, such as free radical damage and skin cancer. Very few sunscreens on the U.S. market would meet the baseline UVA protection standards proposed in Europe (Osterwalder 2009).
While the FDA fails to act on modernizing sunscreen standards and expanding the roster of approved chemicals, Americans continue to be exposed to more UV radiation than ever.
No comments:
Post a Comment